BonoFill
![]()
A regenerative medicine advanced therapy product candidate of a live human bone graft for treating bone defects based on the patient’s own cells, grown outside their body.
It is currently being evaluated in clinical trials for its potential to repair bone defects in maxillofacial, dental, and orthopedic applications.
BonoFill | Our First Tissue-Engineered Bone
![]()

Versatile tissue-engineered graft designed to replace autologous bone transfer
![]()
With its broad expected applicability, BonoFill is designed to address a potential market of millions of bone grafting procedures. For healthcare providers, it could add more treatment options for complex cases and can potentially reduce surgery time. For payers, BonoFill has the potential to reduce hospitalization and surgery costs.
Clinical trial milestones
![]()
- Favorable Safety Profile: BonoFill has demonstrated a favorable safety profile, with no serious adverse events reported in two Phase II clinical trials.
- Maxillofacial Bone Deficiencies: In a completed Phase II trial evaluating BonoFill for the treatment of facial bone deficiencies, 90% of transplantations resulted in complete bone reconstruction, as defined by the study protocol.
- Orthopedic Indications: In an ongoing Phase II trial for the treatment of critical limb bone defects incapable of self-healing, 24 subjects have been enrolled and treated to date. The trial includes cases involving complex forearm fractures, critical-sized tibial defects, and valgus knee deformity repairs, in which bone regeneration and functional rehabilitation have been observed.
Versatile tissue-engineered graft designed to replace autologous bone transfer
![]()
With its broad expected applicability, BonoFill is designed to address a potential market of millions of bone grafting procedures. For healthcare providers, it could add more treatment options for complex cases and can potentially reduce surgery time. For payers, BonoFill has the potential to reduce hospitalization and surgery costs.

Clinical trial milestones
![]()
- Favorable Safety Profile: BonoFill has demonstrated a favorable safety profile, with no serious adverse events reported in two Phase II clinical trials.
- Maxillofacial Bone Deficiencies: In a completed Phase II trial evaluating BonoFill for the treatment of facial bone deficiencies, 90% of transplantations resulted in complete bone reconstruction, as defined by the study protocol.
- Orthopedic Indications: In an ongoing Phase II trial for the treatment of critical limb bone defects incapable of self-healing, 24 subjects have been enrolled and treated to date. The trial includes cases involving complex forearm fractures, critical-sized tibial defects, and valgus knee deformity repairs, in which bone regeneration and functional rehabilitation have been observed.
Representative Images from Phase II Clinical Trial Evaluating BonoFill in Maxillofacial Bone Reconstruction

Baseline X-ray image from a Phase II clinical trial evaluating BonoFill in maxillofacial bone reconstruction. Pre-transplant, the patient’s average residual bone height was 6.6 mm
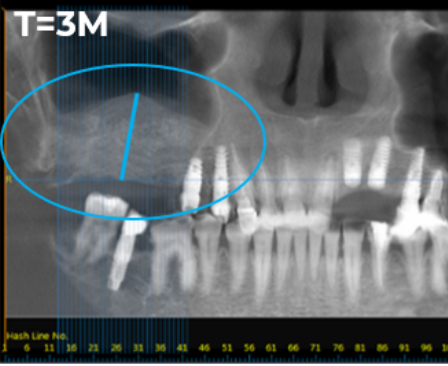
Three-month follow-up X-ray from Phase II clinical trial. Radiological assessment measured an average bone height of 14.8 mm in treated patient.
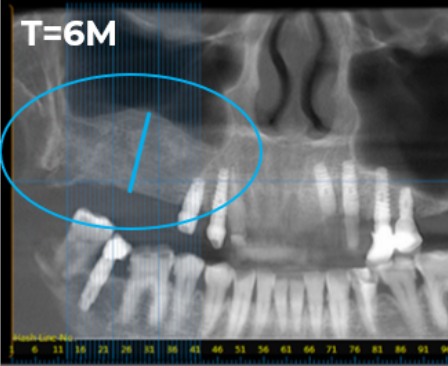
Six-month follow-up X-ray from Phase II clinical trial. Radiological assessments reported an average bone height of 15.6 mm in treated patients.
Additional examples include the potential use of BonoFill for sinus augmentation or for filling bone voids following cyst removal.
Individual patient outcomes may vary, and actual results may differ. These applications are investigational and remain subject to further clinical research.
Tzur, E. et al. (2021). Journal of Oral and Maxillofacial Surgery, 79(4), 787-798.e2.
Examples from a Clinical Trial to Treat Critical Bone Defects in the Limbs using BonoFill


Additional examples include the potential use of BonoFill for joint fusion procedures.
Individual patient outcomes may vary, and actual results may differ. These applications remain subject to ongoing clinical evaluation and further research.
Patient Case from Clinical Research Evaluating BonoFill
![]()
Danny Yakobson, an athlete who suffered a severe leg injury, participated in a clinical trial evaluating BonoFill for bone reconstruction. The investigational treatment involved transplanting lab-grown bone derived from his own cells. Clinical follow-up assessed the integration of the graft, and Danny later participated in the ISRAMAN 13 months post-procedure.
Individual patient outcomes may vary, and actual results may differ. Further research is ongoing.





